Blog ini disediakan demi kemajuan perawat. Kirimkan saran dan kritik Anda ataupun tulisan dan foto/video tentang keperawatan dan kesehatan ke : ppnibontang@gmail.com atau ppni_bontang@yahoo.co.id Terima kasih.
Senin, 21 Desember 2009
LUAR BIASA !!! 3,3 JUTA PER PESERTA
Sabtu, 31 Oktober 2009
PERDICI End Year Symposium
PERDICI END YEAR SYMPOSIUM
With The Theme “From Research to The Best Clinical Practice”
JW Marriot Hotel Surabaya, 04 – 08 December 2009
Organizad By Indonesian Society Of Intensive Care Medicine (ISICM)
AGENDA:
A. PRE SYMPOSIUM COURSE (CEP),THURSDAY – FRIDAY, 03 – 04 DECEMBER 2009
CEP 2 DAYS OPTIONS:
- Workshop on Mechanical Ventilatory: From Basic Theory to Clinical Practice
- Management of Infection in Critically Ill Patients: Practical Approach and Guideline (2009 Updated Curriculum)
- Fluid Therapy: Monitoring and Management
CEP 1 DAY OPTIONS:
- Supporting Laboratory Data Intepretation
- Acid Base Balance and Electrolyte – Steward Approach (2009 Updated Curriculum)
B. NATIONAL SCIENTIFIC MEETING & EXHIBITION
END YEAR SYMPOSIUM & EXHIBITION, SATURDAY – SUNDAY05 – 06 DECEMBER 2009
- Advanced Scientific Lectures & Thematic Sessions
- Basic ICU Nursing Section
C. FUNDAMENTAL CRITICAL CARE SUPPORT (FCCS), MONDAY – TUESDAY, 07 – 08 DECEMBER 2009
AUDIENCE:
ANTICIPATED MORE THAN 400 ATTENDEES FROM RELATED DEPARTMENTS SUCH AS: Intensivist, Anesthesiology, Neuro Surgery, Internal Medicine, Cardiology, Surgery, Pulmonology, Digestive Surgery, Emergency & ICU Doctors, Pediatric, General Practitioners, Neurology, ICU Nursing, Clinical Pathology, Clinical Microbiology
Jumat, 25 September 2009
Mencegah dan Mengelola Mendelson’s syndrome
Volum dan derajat keasaman asam lambung menentukan keparahan akibat aspirasi. Aspirasi dapat dicegah dengan puasa pra pembedahan, pemberian obat-obatan untuk mengurangi volum dan keasaman lambung dan melakukan teknik anestesi yang tepat.
Tindakan segera yang dilakukan setelah diduga terjadi aspirasi adalah tindakan suportif dengan memposisikan kepala pasien lebih rendah dari tubuhnya (head down), pembersihan jalan napas, diberikan oksigen 100% dengan PPV. Dengan melakukan obervasi keadaan klinis dalam 2 jam setelah aspirasi, ditentukan apakah pasien perlu dilakukan tindakan lanjutan di ruang perawatan intensif. Terapi oksigen dan pemberian bronkodilator disesuaikan dengan keadaan klinis dari pasien tersebut. Pemberian antibiotika dilakukan apabila pasien sudah dinyatakan pneumonia. Pemberian kortikosteroid hanya bermanfaat apabila aspirat asam lambung pHnya berkisar antara 1,5-2,5.
Salah satu penyulit selama tindakan pembedahan dengan pembiusan umum adalah terjadinya aspirasi. Dalam konteks ini yang dimaksud dengan aspirasi adalah masuknya benda asing melalui trakea ke paru. Benda asing tersebut dapat berasal dari lambung, esofagus, mulut dan hidung, serta dapat berupa makanan, darah, air ludah atau cairan lambung. Masuknya cairan lambung ke saluran napas dapat terjadi akibat muntah atau regurgitasi.
Mendelson’s syndrome merupakan kejadian yang sangat dikhawatirkan oleh anestetis karena dapat mengancam jiwa pasien. Warner et al. membuktikan, 3 bahwa dari 215.488 tindakan pembiusan umum berisiko terjadi Mendelson's Syndrome, angka kejadian aspirasinya adalah 1:3886 untuk pembedahan elektif, dan 1:895 untuk pembedahan darurat. Enam puluh empat persen pasien yang mengalami aspirasi dalam waktu 2 jam tidak tampak gejala pada proses pernapasan, akan tetapi terjadi kematian pada tiga pasien dari enam pasien yang dilakukan pemasangan alat bantu napas (respirator) dalam 24 jam.
Pasien yang mengalami aspirasi harus didiagnosis dengan tepat dan cepat agar dapat dilakukan penanganan yang adekwat sesegera mungkin. Gejala terjadinya aspirasi harus dapat cepat diidentifikasi. Mendelson mengklasifikasikan 2 kelompok gejala akibat aspirasi dari isi lambung. Kelompok pertama adalah gejala akibat dari bahan padat isi lambung yang mempunyai tanda dan gejala sianosis, wheezing, coughing, takhipneu, hipotensi dan mediastinal shift dan konsolidasi jaringan paru. Kelompok kedua adalah gejala dikenal dengan sindroma Mendelson yang klasik, adalah akibat dari aspirasi asam dengan gejala spasme bronchus, takhipneu, wheezing, sianosis dan panas.
Berikutnya sejak diketahui bahwa aspirasi lebih mudah terjadi pasien obstetri, Mendelson menyatakan bahwa penyebab aspirasi antara lain adanya perubahan anatomi dan fisiologi pada ibu kehamilan, pengosongan lambung yang memanjang dan penurunan kekuatan otot sphincter-esophagus.
Aspirasi isi lambung dapat dan harus dihindari oleh tim pembedahan, oleh karena itu sebagai anggota tim seorang anestesiologis memerlukan penguasaan pengetahuan tentang apirasi ini dan ketrampilan teknik mengatasinya. Dalam naskah ini akan diuraikan secara singkat tentang patofisiologi, predisposisi, pencegahan dan penatalaksanaan/manajemen aspirasi.
Patofisiologi
Aspirasi isi lambung, penyebab, akibat dan gejalanya dapat dibedakan oleh 3 bahan aspirat jaitu berupa asam, partikel (sisa makanan) dan bakteri. Secara umum aspirasi dapat dicegah dengan menjaga isi lambung agar tidak masuk ke esophagus dan faring, aspirat yang di faring dijaga tidak masuk trakhea dan paru. Selain bahan aspirat, volume isi lambung menentukan keparahan akibat aspirasi sehingga jumlah yang cairan masuk paru diupayakan menjadi lebih sedikit.
Timbulnya reaksi akibat aspirasi asam dapat terlihat segera setelah kejadian atau gejala yang timbulnya lambat. Aspirasi asam lambung terjadi 2 fase yaitu trauma pada jaringan dan reaksi keradangan. Dalam waktu 5 detik, asam akan bereaksi dengan mukosa trakhea dan alveoli, dan dalam waktu 15 detik telah terjadi netralisasi. Enam jam kemudian akan kehilangan lapisan sel superfisial yang bersilia dan yang tidak bersilia. Regenerasi terjadi dalam waktu 3 hari, dan dalam waktu 7 hari terjadi regenerasi yang sempurna pada sel yang mengalami kerusakan. Sel alveolar tipe II sangat peka terhadap asam hidroklorid dan mengalami kerusakan dalam waktu 4 jam setelah terjadinya aspirasi. Peningkatan yang cepat lisophophosphatidyle choline dalam 4 jam setelah aspirasi asam mengakibatkan peningkatan permiabilitas alveolar dan cairan paru (lung water). Peningkatan cairan paru mengakibatkan menurunkan compliance paru, meningkatkan ventilation-perfusion mismatching dan meningkatkan alveolar-arterial oxygen tension difference. Pada fase kedua, ditandai dengan acid-mediated induction dan pelepasan pro-inflamatory cytokine seperti TNFα dan interleukin-8. Hal ini akan merangsang ekpresi sel adhesion molecule L-selectin dan beta-2 integrins pada neutrofil, and intercellular adhesion molecules (ICAM) pada endothel paru yang selanjutnya merangsang reaksi keradangan (neutrophilic inflammatory response).
Aspirasi lokal memicu reaksi keradangan yang menyeluruh yang memungkinan terjadinya kegagalan kardiopulmoner. Aspirasi isi lambung secara bersama dengan adanya akibat partikel, menyebabkan terjadi fokus keradangan dan reaksi tubuh terhadap benda asing dengan kerusakan jaringan secara menyeluruh akibat asam. Partikel dan asam lambung bekerja sama secara sinergis menyebabkan kebocoran kapiler alveolar
Aspirasi partikel besar dari isi lambung, akan menimbulkan gejala obstruksi jalan napas, dan dalam waktu pendek dapat terjadi kematian pasien, oleh karena itu partikel tersebut harus segera dikeluarkan, dan dilakukan oksigenasi dan ventilasi untuk menghindari hipoksia, dan segera dilakukan intubasi untuk mencegah aspirasi selanjutnya.
Isi lambung tidak steril sehingga aspirasi yang terjadi dapat disertai bakteri. Enam puluh sampai 100% terdiri dari kuman anaerob. Gabungan kuman aerob dan anaerob sering dijumpai pada aspirasi pneumoni yang terjadi di rumah sakit. Pseudomonas aeroginosa, Klebsiella dan Escheresia colli merupakan kuman gram negatif yang banyak dijumpai sebagai penyebab pneumonia nosokomial. Staphylococcus aureus merupakan kuman gram positif yang patogen. Kuman gram negatif yang dijumpai pada pemakaian ventilator, 34% berasal dari aspirasi isi lambung dan sekret orofaring, dan diduga merupakan penyebab kematian pneumonia pasca bedah.
Predisposisi terjadinya aspirasi.
Meningkatnya kejadian aspirasi, disebabkan oleh adanya faktor pasien, faktor pembedahan, faktor anestesi.
Faktor pasien
Adanya peningkatan isi lambung, seperti yang terjadi pada: 1) pasien dengan hipersekresi lambung pada kehamilan dan obesitas, 2) pembedahan emergensi yang waktu puasanya belum cukup, dan 3) pengosongan lambung yang memanjang terjadi pada kehamilan, obesitas, trauma, pemberian opioid, kelainan gastro intestinal (obstruksi usus, hambatan pada proses pengeluaran dari lambung dan pada perdarahan saluran pencernakan atas), neuropati anatomi karena diabet dan kegagalan ginjal meningkatkan kemungkinan aspirasi.
Selain itu kondisi seperti: 1) meningkatnya kecenderungan terjadinya regugurgitasi terjadi pada pasien dengan tonus sphincter esophagus yang menurun, misalnya pada kehamilan, obesitas dan hiatus hernia, adanya reflek gastro esophageal yang terjadi pada hiatus hernia dan kelainan GIT, kelainan esophagus misalnya penyempitan dan carcinoma esophagus dan pada usia lanjut; serta 2) inkompenten dari laring misalnya pasien yang tidak sadar, kelainan anatomi laring dan neuro muscular disorder akibat bulbar atau pseudobulbar palsy juga dapat meningkatkan kemungkinan aspirasi.
Faktor pembedahan
Tehnik pembedahan yang harus diperhatikan berkaitan dengan kemungkinan terjadinya aspirasi diantaranya adalah: 1) manipulasi usus pada pembedahan abdomen atas dapat terjadi reflux dari lambung, 2) kenaikan tekanan intra-abdominal akibat pneumoperitoneum pada pembedahan laparoscopi, dan 3) posisi lithotomy dan trendelenburg mendesak gaster ke arah proksimal menyebabkan terjadi regurgitasi.
Faktor anestesi
Hal yang dapat menyebabkan kemungkinan terjadinya aspirasi sewaktu melakukan anestesi yang perlu diantisipasi adalah: (1) pemberian obat anestesi lokal disekitar trakhea pada saat intubasi, (2) belum kembalinya kemampuan batuk akibat pemberian obat pelumpuh otot (neuroblocking agent), (3) teknik anestesi yang tidak sesuai misalnya a) laringoskopi yang dilakukan pada tahap anestesi yang dangkal dan sudah diberikan obat pelumpuh otot menyebabkan timbulnya batuk, regurgitasi dan muntah, b) dilakukan ekstubasi sebelum kembalinya reflek untuk melindungi jalan napas dari muntah dan regurgitasi c) pemakaian laryngeal mask atau alat pembebas jalan napas yang berada di depan supraglotik menyebabkan penurunan tonus spinkter esofagus distal, serta (4) kesulitan manajemen jalan napas, misalnya kesulitan melakukan intubasi sehingga harus memberikan positif pressure ventilation dengan masker, cricoid pressure yang tidak sempurna atau melepaskan tekanan sebelum endotracheal tube nya masuk dan pemasangan endotracheal tube yang masuk ke esofagus pada pasien dengan lambung penuh.
Verghes melakukan audit prospektif pada 2359 pasien yang dilakukan anestesi umum dengan LMA, 41% dilakukan positve pressure ventilation 5 pasien mengalami regurgitasi, 3 pasien terjadi pada saat pelepasan LMA pasca bedah, hanya 1 pasien yang diketahui terjadi regurgitasi dan pasca bedah tidak ada gejala sisa.4 Hasil survei Brimacombe dan Berry di unit perawatan intensive pada tahun 1990-1991 memperlihatkan bahwa 758 pasien dengan laryngeal mask, 8 pasien terjadi aspirasi ada 1 orang terjadi aspirasi penumonitis, tanpa ada kematian.
Pencegahan aspirasi
Aspirasi dapat terjadi setiap saat, sebelum, selama dan sesudah pemberian anestesia. Aspirasi isi lambung tanpa gejala terjadi pada 45% pasien tidur dan 75% pada pasien tidak sadar. Kecurigaan terjadi aspirasi apabila terdengar suara tambahan, terjadi kenaikan airway pressure atau pengeluaran sekret yang berlebih. Adanya suara napas tambahan berupa wheezing, rales yang menyeluruh, takhipneu, takhikardia, dan panas yang tidak tinggi merupakan gejala dari aspirasi isi lambung. Untuk diagnosa pasti dapat dilakukan dengan pemeriksaan invasif misalnya fibreoptic bronchoscopy, bronchoalveolar lavage.
Aspirasi dapat terjadi pada keadaan peningkatan tekanan lambung, meningkatnya kecenderungan terjadinya regurgitasi dan adanya penurunan kompetensi laring. Pencegahan dilakukan dengan mengurangi produksi asam lambung dan keasaman lambung. Produksi asam lambung yang lebih dari 25ml(0,4 ml/kg) dan pH kurang dari 2,5 mempunyai resiko yang lebih besar. Apabila pH asam lambung kurang dari 1,5, kerusakan yang terjadi pada paru sangat hebat.
Aspirasi dapat dicegah melalui: puasa pra pembedahan, pemberian obat-obatan untuk mengurangi volume dan keasaman lambung dan melakukan dengan teknik anestesi yang tepat.
Puasa pra pembedahan
Puasa merupakan salah satu cara dari pengurangan isi lambung yang berupa padat dan cair. Berkurangnya jumlah asam lambung akan meminimalkan efek terjadinya aspirasi pneumonitis. Tujuan utama puasa adalah mengurangi volume isi lambung dibawah 25 ml. Puasa pra bedah menyebabkan mulut kering, haus, meningkatkan resiko PONV dan terjadinya hipovolemi. Pengosongan cairan lambung dikendalikan oleh bagian proksimal dari gaster dan berkaitan langsung perbedaan tekanan dari gastroduodenal, kecuali kalau ada hal yang patologi dari pyloric dan terjadi perubahan anatomi akibat pembedahan.
Jumlah isi lambung tergantung dari dimulainya waktu puasa. Puasa dengan minum air putih 2 jam sebelum pembedahan tidak meningkatkan volume cairan lambung dan keasaman lambung, karena dalam 2 jam sudah terjadi pengosongan lambung, tetapi apabila minum ASI pengosongan lambung baru terjadi setelah 4 jam. Untuk susu formula, makanan ringan pasien dipuasakan dalam waktu 6 jam. Makanan berat pengosongan lambung terjadi dalam waktu 9 jam.
Pemberian obat-obatan untuk mengurangi volume dan keasaman lambung
Derajat keasaman lambung sangat berpengaruh terhadap derajat kerusakan dan kegawatan dari aspirasi paru. Pemberian Na citrate akan meningkatkan pH asam lambung. Sucralfate akan mengikat empedu dan asam lambung, mempunyai efek untuk perdarahan lambung, tetapi apabila terjadi aspirasi akan menyebabkan pneumonitis akut dan perdarahan paru. H2 reseptor antagonis yang diberikan 90-120 menit akan mengurangi produksi dan menaikkan pH asam lambung. Apabila pasien sudah memakai obat H2 reseptor antagonis untuk beberapa waktu efektivitas untuk mengurangi produksi dan menaikan pH asam lambung berkurang sehingga akan meningkatan resiko akibat terjadi aspirasi paru. Proton pump inhibitors (PPls) mengikat residu cisteine dari H+/K+ ATPase pump mukosa gaster. PPls menurunkan produksi dan meningkatkan pH asam lambung, namun efek tersebut tidak tampak menurunkan kejadian dan keparahan dari paru akibat aspirasi.
Obat prokinetic yang dikenal dengan metoclopramide menurunkan resiko aspirasi dengan menurunkan volume isi lambung. Efek obat prokinetic akan dihambat oleh atropin (10µg/kg), pemberian opiat yang menyebabkan perpanjangan pengosongan lambung dan meningkatkan tonus dinding lambung.
Teknik anestesi
Aspirasi paling sering terjadi pada saat induksi dan laringoskopi. Kemungkinan terjadinya aspirasi ini dapat dikurangi dengan mengisolasi jalan napas dengan tractus gastrointestinal. Pemasangan endotrakheal secara sadar atau dilakukan rapid sequence induction dengan cricoid pressure akan mengurangi terjadinya aspirasi. Sellick mengemukakan dengan dilakukan penekanan pada cricoid pada pasien yang telentang dan kepala lebih rendah(slight head down) akan mengakibatkan isi lambung yang keluar tidak dapat masuk dalam jalan napas. Posisi head up 450 pada saat intubasi untuk menghindari terjadinya aspirasi. Laringokopi yang dilakukan dengan kedalaman anestesi yang tidak cukup akan mengakibatkan batuk, bucking, muntah dan spasme laring. Keadaan ini akan menyulitkan intubasi sehingga akan memperbesar kemungkinan terjadi aspirasi.
Pemakaian LMA tidak mengisolasi jalan napas dengan tractus gastrointestinal. Hasil meta analisis menunjukkkan bahwa 2 dari 10.000 yang dilakukan dengan LMA mengalami aspirasi.
Manajemen aspirasi
Aspirasi merupakan resiko dari tindakan anesthesia dan pemberian obat-obatan yang mengurangi reflek proteksi jalan napas. Aspirasi dapat menyebabkan pneumonitis, meningkatkan kejadian pneumonia dan adult respiratory distress syndrome (ARDS).
Tindakan segera setelah diketahui terjadi aspirasi, pertama adalah terapi suportif dengan, pasien diposisikan head down untuk meminimalkan kontaminasi isi lambung dengan paru. Mulut dan faring segera dibersihkan dengan menekan cricoid. Pembersihan jalan napas melalui endotrakheal dapat dilakukan dengan mengisap intratrakheal yang sebelumnya diberikan oksigen 100% dengan PPV. Tindakan selanjutnya adalah melakukan bronhoscopy untuk membuang partikel dari aspirat. Pemasangan oro/nasogastro ditujukan untuk mengosongkan lambung dan mengukur derajat keasaman lambung. Terapi oksigen dan bronchodilator diberikan sesuai dengan keadaan klinis dari pasien tersebut.
Setelah diagnosis aspirasi ditegakkan kelanjutan dari tindakan pembedahan dapat dibicarakan dan disesuaikan dengan keadaan pasien. Setelah pembedahan berakhir dilihat keadaan klinik dalam 2 jam setelah aspirasi, apakah pasien perlu dilakukan tindakan lanjutan di ruang perawatan intensif. Pertimbangan ini perlu dilakukan untuk menyelamatkan jiwa pasien. Warner melakukan studi retrospektif pada 66 pasien yang mengalami aspirasi. Empat puluh dua pasien dari 66 orang dalam 2 jam tidak tampak adanya gejala dan pasca bedah tidak dilakukan intervensi pada pernapasan. Delapan belas pasien yang dilakukan rawat jalan 12 pasien, pulang pada hari tersebut. Delapan belas pasien dari 24 pasien yang menunjukan gejala wheezing, penurunan SpO2 lebih dari 10% dan ada gambaran radiologis dari aspirasi dalam waktu 2 jam. Pasien tersebut dilanjutkan perawatan di ICU untuk diberikan napas buatan. Tiga pasien dilakukan napas buatan lebih dari 24 jam, dan 2 orang mengalami sindroma distres napas dan meninggal.
Pemberian antibiotika dilakukan bila pasien sudah dinyatakan pneumonia. Pemeriksaan mikrobiologi dari pasien aspirasi diperlukan untuk memastikan pemberian obat-obatan. Bahan aspirat membawa kuman masuk kedalam jaringan paru. Dari penelitian bahan aspirat pada kasus aspirasi berat, didapatkan kuman basili gram negatif 49%, bakteri anaerob 16% dan stafilokokus 12%. Keberadaan kuman basili gram negatif menunjukan bahwa pasien tersebut mengalami aspirasi dari bahan tractus gatrointestinal.
Pemberian corticosteroid masih kontroversi. Pertimbangan penggunaannya adalah untuk mengurangi keradangan dan stabilisasi membrane lysosom. Selain itu diduga dapat mencegah kerusakan sel paru dengan cara melindungi pneumosit alveolar tipe II dan mengurangi aglutinasi leukosit dan platelet. Hasil penelitian eksperimental oleh Downs JB et al menunjukan efektivitas pemberian kortikosteroid ada hubungannya dengan nilai pH Aspirat, jika pH aspirat berada pada 1,5-2,5 terapi corticosteroid berperan untuk membantu proses kesembuhan acid aspiration pneumonitis. Dexamethasone diberikan 0,8 mg/kg BB tiap 6 jam menurunkan cairan paru (lung water) secara bermakna mulai 24jam, dan kembali kekeadaan normal setelah 72 jam. Bila pH aspirat lebih kecil dari 1,5 akan terjadi kerusakan parensim paru yang hebat dan luas, oleh karena itu terapi steroid tidak efektif. Apabila pH aspirat lebih besar dari 2,5 pemberian kortikosteroid tidak ada artinya. Penelitian Wolfe et al, memperlihatkan bahwa pasien pneumonia pasca aspirasi yang disebabkan oleh kuman gram negatif lebih banyak ditemukan pada pasien yang diberi kortikosteroid.
Jumat, 07 Agustus 2009
Perioperative Dental Considerations for the Anesthesiologist
Jeffrey S.Yasny, Dds From the Department of Anesthesiology, The Mount Sinai School of Medicine, New York. Address correspondence and reprint requests to Jeffrey S. Yasny, DDS, Department of Anesthesiology, One Gustave L. Levy Place, Box 1010, New York, NY 10029-6574. Address e-mail to jeffrey.yasny@mountsinai.org.
Anesthesia & Analgesia 2009; 108(5): 1564-73
Abstract
Although anesthesiologists consistently work in the mouth of patients, they may not have been exposed to a comprehensive education of teeth, surrounding tissues, and intraoral prostheses. Since perioperative dental damage is one of the most common anesthesia-related adverse events and is responsible for the greatest number of malpractice claims against anesthesiologists, several dental considerations are warranted. The likelihood of perioperative dental trauma increases with the vulnerability of a patient’s dentition and the presence of associated anesthesia risk factors. Minimizing dental injuries begins with the anesthesiologist’s preoperative assessment of the patient’s dentition and intraoral tissues. Clear documentation of the patient’s preoperative dental condition and notifying the patient of the potential dental damage will diminish costs for any related postoperative dental treatment. Upon discovery of a potentially hazardous dental condition, a consultation with a dentist should be considered before proceeding with the surgical procedure. Exercising cautionary measures during provocative events, such as laryngoscopy and tracheal extubation, can aid in the prevention of dental trauma. In the event of such an injury, several management tactics can promote a swift and reasonable resolution. Establishing an increased awareness of intraoral conditions and the related perioperative risk factors may diminish the incidence of dental damage and financial costs.
Table of contents
- Incidence and morbidity
- Dental nomenclature, dental anatomy, and pathophysiology of dental injury
- Preoperative evaluation
- Preoperative discussion and documentation
- Recommendations for prevention of perioperative dental damage
- Management plan: when dental damage does occur
- Conclusions
- Footnotes
- References
Although anesthesiologists consistently work in the mouth of patients, they may not have been exposed to a comprehensive education of teeth, surrounding tissues, and intraoral prostheses. To more fully appreciate a patient’s dentition, this article presents pertinent nomenclature and anatomy and discusses the presentations of various vulnerable dentitions and the likelihood of dental trauma. A thorough preoperative assessment of the patient’s dental status, including the recognition of vulnerable teeth, soft tissues, and associated anesthesia risk factors, are of paramount importance in the prevention of perioperative dental damage. This article reviews the incidence, morbidity, pathophysiology, and predisposing risk factors associated with such an injury. For select scenarios, the value of an anesthesiologist performing a more extensive preoperative evaluation is described. The importance of using clear discussion and detailed documentation for the purpose of reducing postoperative distress of all parties involved in the patient’s care is reviewed. Special considerations for the pediatric and adolescent patient populations are also discussed. Several recommendations for the prevention of perioperative dental damage and a plan for its management are presented. Exercising an effective risk reduction strategy for these unfortunate injuries can minimize expenses while maximizing anesthetic outcome and patient satisfaction.
Incidence and morbidity
Return to the table of contents
Based on retrospective data, the incidence of perioperative dental damage has been found to range from 0.02% to 0.07%.1–3 However, a prospective study has reported a much higher incidence. Chen et al.4 examined the dentitions of patients before and after undergoing endotracheal anesthesia and found that the incidence of dental damage was 12.1%. Lockhart et al.1 surveyed 133 directors of anesthesiology training programs and reported an average incidence of 1:1000 dental injuries during or after 1,135,212 tracheal intubations in 1 yr. These data were reinforced by a review of general anesthetics performed by anesthesia residents in which the incidence of dental injury was found to be 0.1%. It was also found that the level of anesthesia resident training does not affect the risk of dental injury.5
Perioperative dental damage is the most common of all medicolegal complaints related to anesthesia, comprising one third of all medicolegal anesthetic claims.6–12 It is also the adverse event responsible for the greatest number of malpractice claims against anesthesiologists.2,7 This is likely due to the clear causative link between an anesthetic and the damaged dentition. The anesthesiologist is often immediately aware once a dental injury occurs, and, because of its highly sensitive and visible location, patients or their relatives notice the injury soon after the anesthetic. Newland et al.3 found that 86% of all dental injuries were discovered by the anesthesia provider, whereas 14% were reported by the patient. Although the financial implications of dental damage during anesthesia are not especially significant per incident, payments for the repair of a dental injury can be reported and become part of the National Practitioner Data Bank.13
In the perioperative period, the majority of dental injuries (50%–75%) occur during tracheal intubation.1,2,7,8,14,15 When a satisfactory view of the glottis is difficult to obtain during laryngoscopy, the patient’s maxillary anterior teeth are sometimes used as a fulcrum by the laryngoscope blade.16 Consequently, the maxillary incisors, particularly the maxillary left central incisor, are damaged most frequently.1,3,6,17 Anterior teeth, such as the incisors, are single rooted with a forward dental axis and a small cross-sectional area, rendering them susceptible to fracture when a strong vertical and/or an oblique force is applied to them.8 Posterior teeth, such as molars, have multiple roots and a wider cross-sectional area and are much better equipped to withstand such forces. The anatomic advantage of posterior teeth and their remoteness from the laryngoscope during intubation contributes to their lower incidence of perioperative trauma in comparison with the anterior teeth.
Aside from laryngoscopy, dental damage can be caused by other events: aggressive suctioning in the posterior of the mouth6; oropharyngeal airway placement subjecting anterior teeth to extreme lateral forces1,6–8; and biting down vigorously upon the endotracheal tube or laryngeal mask airway (LMA) shaft in situ during emergence from anesthesia.18 Other provocative events include the forceful removal of an oral airway, endotracheal tube, or LMA upon emergence, and shivering during the recovery phase which may cause spasm of the masseter muscle leading to excessive pressures while clenching and/or grinding of teeth.7,8,19 Approximately 9%–20% of anesthesia-related dental injuries occur during tracheal extubation or in the recovery room.1,2,4,6,8,14,17,20,21 In addition to the variance in the causative events, the type of tooth injury varies as well. Enamel fracture and loosening/subluxation of a tooth were found to represent 55.2% of all injuries, followed by tooth avulsion (9.0%) and crown fracture (7.7%).3
Return to the table of contents
Dental nomenclature, dental anatomy, and pathophysiology of dental injury
Return to the table of contents
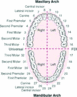
The complete adult (permanent) dentition includes 32 teeth, supported by two opposing arches of bone: the upper jaw (maxilla) and the lower jaw (mandible). The dentition is further divided into four quadrants of up to eight teeth each. The posterior of each quadrant has the potential for three molars and two premolars (bicuspids), whereas in the anterior there is one canine, one lateral incisor, and one central incisor. In the United States, the Universal Numbering System is used (Fig. 1). Teeth are numbered from 1 through 32, counted sequentially whether they are present or missing.
Numbering of the teeth is based as if one were directly facing the patient, beginning at the maxillary right quadrant’s third molar (#1) and sweeping in a clockwise fashion through the maxillary left then mandibular left quadrants and ending at the mandibular right third molar (#32). A child’s dentition (also known as "primary" or "deciduous") consists of a maximum of 20 teeth. Each quadrant is comprised of two molars, a canine, a lateral incisor, and a central incisor. In the United States, each "baby" tooth is designated a letter from A through T. In many other countries, the Federation Dentaire Internationale system is commonly used. In this system, each tooth is designated a specific two-digit number based upon two components. The first digit denotes its specific quadrant, determined by a clockwise arrangement, permanent dentition (#1–4) or primary dentition (#5–8). The second digit refers to the tooth’s location from the midline of the dentition. For example, the permanent mandibular right first molar is designated as tooth #46 (i.e., it is situated in the fourth quadrant and is the sixth tooth from the midline).
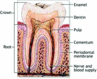
The tooth is divided into two parts, the crown and the root, each consisting of three layers (Fig. 2). Enamel is the outer layer of the crown that becomes fragile if not supported by viable dentin. Dentin is the middle layer, yellowish in color, and provides the framework of the tooth. The pulp is the innermost layer and consists of blood vessels and nervous tissue.22 Cementum is the outer layer of the root. Dental caries is the most common disease affecting teeth. The process involves bacteria adhering to a tooth and producing acids that decalcify and undermine the enamel.23 As the decay develops further and encroaches upon the pulp, sensitivity is noted by the patient. A deep carious lesion may require endodontic (root canal) therapy. A tooth becomes proportionately more vulnerable to injury as its natural structure becomes more compromised. Treatment of caries involves removal of the decayed portion of the tooth and the placement of a dental restoration (filling),24 (Table 1) producing a tooth that is physically weaker and more prone to injury.4,8,20
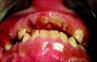
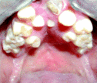
Patients who present with decayed or restored teeth are classified as having a preexisting dental condition. This category also includes individuals having significant periodontal disease, an inflammatory process involving a bacterial infection of the periodontium.25 The periodontium is the tissue that surrounds the tooth and provides it with support, and is comprised of gingiva (i.e., gums) and the underlying alveolar bone and periodontal ligament.26,27 Periodontal disease commonly manifests in an adult’s mouth as inflamed gingiva, gingival recession, and calculus (i.e., tartar) accumulation (Fig. 3). Dissolution of the periodontium leads to increased tooth mobility and ultimately to a dentition that has increased vulnerability to subluxation or avulsion when subjected even to slight forces.1,4,7,20
In the perioperative period, dental damage results from the coupling of a preexisting dental condition with a physical event, such as pressure or forces applied to a tooth. Newland et al.3 found that patients with poor dentition or reconstructive work were 3.4 times more likely to have dental injuries related to anesthesia. In a retrospective analysis of incident reports of dental injury for elective intubations, 72% of the incidents occurred in patients aged 50–70 yr, likely due to the higher incidence of periodontal disease in that group.6 An increased incidence of dental injury has also been reported in cases exhibiting the following anesthesia risk factors: general anesthesia, endotracheal intubation, emergency surgery, and a difficult airway (i.e., Mallampati Class 3 or 4).28 The combination of a preexisting dental condition with any of the aforementioned anesthesia risk factors results in a dentition that is even more vulnerable to damage.14
Return to the table of contents
Preoperative evaluation
Return to the table of contents
During the preoperative assessment, an essential focus for the anesthesiologist is the patient’s airway, including dentition. Determining the susceptibility of any loose teeth and taking appropriate precautions to avoid dental damage is necessary but not always sufficient. In certain instances, it would be beneficial for the anesthesiologist’s attention to extend beyond asking a patient to open his or her mouth and protrude the tongue, or whether there are any loose teeth, crowns, or dentures. Using a more extensive evaluation of the patient’s intraoral condition is rarely exercised by anesthesiologists, and it is surely not indicated for the vast majority of patients given the time constraints of clinical practice. However, although the suggestion to incorporate this component into one’s preoperative evaluation may seem extreme, there are particular cases in which its application would be valuable.
For example, when asked by the anesthesiologist to open their mouth, some patients may demonstrate an obviously poor dentition and have extremely mobile teeth that are at risk for avulsion and aspiration during the perioperative period. Since chronic dental neglect exists in some individuals, upon their arrival for a surgical procedure they may also be harboring an unknown odontogenic infection. An untreated dental abscess that is not discovered preoperatively can contribute to unplanned and extended postoperative treatment, increased expenses, and a compromised surgical outcome. Therefore, given that it may be an anesthesiologist who is the first caregiver to look inside a patient’s mouth in years or even decades, a more thorough examination of such patients’ airways may be worthwhile.
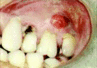
If the anesthesiologist is suspicious about the existence of an intraoral abscess, a closer examination is warranted (Fig. 4). Notable redness, swelling, purulent discharge, or fistulas may be signs of an infection and can be visible along the gingiva (Fig. 5). An intraoral abscess can also aggressively tract through bone resulting in an endodontic or periodontal infection that can manifest as an extraoral swelling in the submandibular, submental, or mid-face regions. If a dubious area is detected, a consultation with a dentist should be considered before proceeding with the surgical procedure. In select situations, this small investment by the anesthesiologist during the preoperative evaluation can yield significant improvements in surgical outcome and overall patient health.29
The maxillary central incisors (#8 and #9) are in the most vulnerable position during laryngoscopy, are the most commonly injured teeth, and demand the greatest cosmetic attention. Maxillary anterior teeth tend to be restored with bonding, veneers, or crowns making them even more prone to damage because these restorations are not as resilient as natural teeth. Complicating matters is that porcelain, the restorative material of choice, is not easily recognized as being artificial.
The presentation of a patient’s dentition is highly variable. Isolated teeth and abutments (i.e., teeth adjacent to an edentulous space that have been reduced to support a removable partial denture) may present with occult mobility and are more susceptible to injury.30 Teeth are sometimes worn excessively by attrition (wear between opposing teeth), physical damage (abrasion), or chemical damage (erosion),31 which may be caused by dietary or gastric acids.32 Chronic use of medications can adversely affect dental and periodontal health. In adults, a plethora of medications, especially those with anticholinergic activity, are the most common cause of dry mouth (xerostomia).33 This condition is also one of the most common complaints following radiation therapy for head and neck cancers34 and leads to hyposalivation-induced rampant caries.35 For such patients and in the elderly, root decay that usually presents along the gingival margins of the teeth (i.e., the junction of where a tooth emerges from the gums) can cause the crown of the tooth to be severed perioperatively.36
When patients present with significantly loose teeth, they are usually aware of their mobility but may not admit it because of embarrassment or their underestimation of the condition’s potentially significant perioperative implications. For any suspiciously susceptible teeth noted preoperatively by the anesthesiologist, it is suggested that he or she put on a glove and slightly wiggle them to better appreciate their mobility.
Pediatric and Adolescent Populations
Young children may present for surgery with an intraoral condition known as early childhood caries or "baby bottle" syndrome. This condition arises from the following sequence of events: To soothe a crying child at night, a parent will give the child a bottle filled with sugar-containing liquids, such as milk or juice. Night after night, repeated bathing of a young child’s teeth in these acid-promoting substances leads to rampant decay.37 The primary maxillary incisors and mandibular molars are directly subjected to the sugary liquids; consequently, they are the teeth that are most commonly affected by this decaying process (Fig. 6). Interestingly, the child’s tongue tends to protect the mandibular incisors from the liquid emanating from the bottle’s nipple which is positioned between the tongue and the palate. This unfortunate yet preventable situation is principally due to a lack of parental education and typically manifests in children between the ages of 18 and 48 mo. For the anesthesiologist such soft eroded enamel is highly vulnerable to crumbling during intraoral manipulation and can become an unexpected event during an intubation.
Eruption of primary teeth usually begins at about 6 mo of age and most children have a complete set of these teeth by the age of 3 yr.38 Primary teeth exhibit long slender roots that are less likely to withstand excessive physical forces, rendering them vulnerable to dislodgement perioperatively. If trauma is sustained to a primary tooth, the development of the underlying permanent successor can be adversely affected.30 The natural exfoliation of deciduous teeth usually commences at the age of 5 or 6 yr when the primary mandibular central incisors are replaced by their permanent analogues. As adult teeth begin to erupt into a child’s mouth, they resorb the roots of the baby teeth that they are succeeding, causing mobility, and eventual exfoliation. This period of tooth turnover commonly occurs during the ages of 5 through 12 yr, producing a "mixed" dentition of primary and permanent teeth. Children in this age group are more susceptible to tooth injury.39 For example, newly erupted permanent incisors may be readily avulsed in children aged 6–8 yr because their immature roots may not fully develop for another 3 yr.30
The presence of any intraoral appliances should also be confirmed during the preoperative assessment of this patient population. Devices used for breaking the childhood habits of tongue thrusting and thumb sucking often suspend from the hard palate and may interfere with laryngoscopy.40 Due to imperfect eruptions, children may exhibit crowding of teeth or an extra (i.e., supernumerary) tooth. A patient’s history of a cleft palate may yield a narrow maxillary arch that can also lead to crowding of teeth upon their eruption in the maxilla (Fig. 7). Adolescents (and adults) may present with orthodontic appliances that are removable, such as a biteplate (retainer) or fixed such as brackets (braces). Another fixed appliance known as a palatal expander is designed to promote widening of the maxilla. This device can limit the space available for a laryngoscope and can increase the likelihood of dental damage or a traumatic intubation (Fig. 8).
Return to the table of contents
Preoperative discussion and documentation
Return to the table of contents
Since dental damage is one of the most likely adverse outcomes during general anesthesia, it is recommended that the patient be made aware of this possibility during the preoperative evaluation, especially with an anticipated difficult intubation and/or a patient’s vulnerable preexisting dentition. Forewarning patients about this potential adverse incident preoperatively can substantially decrease the likelihood of facing an uninformed, unprepared, or angry patient postoperatively.
The preoperative presentation of a poor dentition should prompt the anesthesiologist to be descriptive in documenting this condition. "None loose" or "intact" are not always appropriately illustrative. Also, a notation referring to the patient’s periodontal status can be helpful. For example, "poor oral hygiene with generalized periodontal disease, multiple mobile teeth and partial edentulism in both arches" can succinctly summarize a patient’s dentition that is especially vulnerable to damage. In addition, the following entry made in a patient’s medical record preoperatively can save time disputing such a claim postoperatively: "the maxillary right central incisor (#8) has a fractured incisal edge which I have confirmed with the patient." Any missing, damaged, or loose teeth should be confirmed with the patient and documented accordingly. Gatt et al.41 has proposed the introduction of a standardized uniform dental chart to accurately document the preoperative condition of a patient’s dentition. Detailed documentation of the patient’s preoperative dental condition also serves to minimize the potential for inflated dental treatment estimates following a perioperative dental incident.
Return to the table of contents
Recommendations for prevention of perioperative dental damage
Return to the table of contents
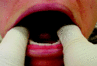
Patients’ loose teeth present the anesthesiologist with the dilemma of having the teeth extracted preoperatively or proceeding with caution. In many instances, it is not practical to obtain a dental consultation and definitive treatment immediately before surgery. Securing a loose tooth is a cautious measure to prevent aspiration and aid in a tooth’s retrieval should it become dislodged. A 3–0 silk suture without the needle can be wrapped several times around the gingival margins of the mobile tooth and adjacent teeth for increased stability (Fig. 9). The suture can be secured with adhesive tape to the ipsilateral cheek and removed after the procedure upon recovery.
The presence of any abnormalities of the tongue, lip, or palate should also be noted. Being cognizant of lesions in any of these areas can reduce perioperative trauma of oral soft tissues. Although all intraoral swellings should be investigated and documented preoperatively, they are not all indicative of an infectious process. For example, a torus is a prominently benign bony growth that can develop in a patient’s palate or mandibular premolar region and would not require preoperative intervention.42 Particularly upon inspection of the patient’s maxillary anterior teeth, biting surfaces should be carefully scrutinized for any evidence of being worn, notched or chipped. Incomplete dental treatment, such as temporary crowns and implants, may become an issue perioperatively and the stability of any such provisional prostheses should be verified. Any removable prostheses (e.g., dentures, orthodontic appliances) or soft tissue piercings of the lip or tongue should be removed, labeled, and stored before the anesthetic induction to prevent any accidental loss or damage.
After induction of anesthesia when a patient’s mouth is being scissored open before laryngoscopy ("cross-finger" maneuver), excessive stresses may be placed upon the mandibular anterior or maxillary right posterior teeth. During this action, one should exercise caution and subject only posterior, not anterior teeth, to minimal vertical or oblique forces to decrease the likelihood of damage. This manipulation can also cause the mandible to "lock" in an open position. This disarticulation is caused by anatomical abnormities or extreme mouth opening forces, resulting in the condyle of the mandible to slide too far anteriorly along the articular eminence of the temporal bone. A simple maneuver can correct this situation. Directly facing the patient who remains under general anesthesia, thumbs can be placed bilaterally along the biting surface of the patient’s mandibular posterior teeth. The action of applying pressure inferiorly and then posteriorly (i.e., down and back) unhinges the condyle from its unnaturally anterior position, and returns the mandible to a more relaxed position (Fig. 10). In a one-sided dislocation, the mandible is deflected ipsilateral to the locked joint. To reduce the disarticulation of the condyle/disk, inferior pressure is applied to the mandible which is then moved gently in the contralateral direction.43
Since laryngoscopy is the most common procedure that may lead to dental damage, prevention of such an injury during this time warrants particular attention. Obviously, a technique involving gentle blade placement and motion, along with carefully applied forces, should be used. One maneuver that minimizes contact with teeth entails placing the right hand on the patient’s occiput and extending the neck, while the left little finger pushes down the chin, opening the mouth and creating access. Careless placement of the laryngoscope blade can cause laceration or abrasion of the lips, palate, and cheeks with possible ulceration and infection.40 Suctioning intraorally should be done with great care, preferably with the use of a 14F soft plastic catheter.
Patients with a Mallampati score of 3 and prominent "buck" teeth have been reported to experience blade-tooth contact in more than 90% of intubations,16 prompting the modification of the laryngoscope blade to avoid dental damage.44 Lee et al.16 reported that using a Macintosh blade with a low-height flange (i.e., Callander modification) reduced the frequency of direct contact between the blade and the maxillary teeth by more than 80%. In contrast, a similar modification of a Miller blade was found to decrease the blade’s effectiveness for laryngeal visualization.45 Angulated blades, such as the McCoy and the Belscope, have been reported to provide greater tooth-blade distances and better visibility than regular curved or straight blades.46,47
Several studies have examined the preventive use of devices that protect teeth during laryngoscopy.48 Various prefabricated or custom-made mouthguards (i.e., those used preventively in sports) do not guarantee an endotracheal intubation free of dental trauma.21 Burton and Baker8 found that the vast majority of anesthesiologists did not use a protective guard routinely, and 45% had never used one. The use of mouthguards has no significant effect on the incidence of dental injury.49 The main disadvantage of these tooth protectors is that their thickness decreases the amount of space within the oral cavity, leading to poor visibility and increased difficulty in guiding the endotracheal tube into the larynx. They also prolong the intubation time, increase the likelihood of oral trauma, and create additional hazards, including aspiration of the appliance. However, for bronchoscopists and endoscopists who tend to use a rigid scope for their procedures, mouthguards have been recommended.49
The oral airway has been found to be a major cause of injury to teeth50,51 and 20% of dental injuries were reportedly caused by Guedel oral airways.18 Oropharyngeal airways should be used with caution for patients with vulnerable anterior teeth and should not be used as a bite block.52,53 Nasopharyngeal airways are a better choice for those patients who are especially at risk for dental injury.30
In preparation for extubation, a soft roll of gauze can be placed on the biting surfaces of the patient’s mandibular premolar/molar region. It should be large enough to be easily retrieved. A bite block can also be made from a wooden tongue depressor, wrapped several times at one end with 1 in. or 2 in. cloth adhesive tape, and inserted with the bundle positioned between the mandibular and maxillary molars on either side of a LMA or opposite to a unilaterally positioned endotracheal tube. When a patient involuntarily bites during emergence from anesthesia, forces will be dissipated throughout stronger multirooted molars rather than weaker single-rooted incisors. Functioning as a fulcrum, this posteriorly positioned roll will also indirectly open the anterior of the mouth, preventing contact and damage to anterior teeth and/or any of their cosmetic restorations upon removal of the airway device. During emergence from anesthesia, the gauze roll will also prevent the patient from clenching down on an endotracheal tube or LMA, which could adversely affect oxygenation. Adequately controlling postoperative shivering will lessen excessive teeth clenching, grinding, or masseter muscle spasm.
Return to the table of contents
Management plan: when dental damage does occur
Return to the table of contents
When an incident of perioperative dental damage occurs, documentation of the injury is imperative. An evaluation of the damage by a dentist should be obtained as soon as possible to determine the extent of the injury and provide potential options for postoperative treatment. Confirming the location and ensuring the successful retrieval of any avulsed or broken teeth is also essential. If a tooth, crown, or other prosthesis is avulsed and its location is unknown, a chest radiograph should be obtained without delay to determine whether it has been aspirated or is on a passage to the stomach. Although most dental fragments will pass through the gastrointestinal tract without causing harm, large prostheses have the potential to obstruct and perforate.39 If the object has not been retrieved, intraoperative intervention may be necessary. The ingestion of a fixed partial denture followed by subsequent recovery with endoscopy during general anesthesia has been reported.54
If a permanent tooth is displaced from its socket, it should be stored in normal saline or cool fresh milk until it can be splinted or reimplanted.39 The success of reimplantation of an avulsed tooth after a traumatic intubation is primarily determined by the elapsed time since injury. If reimplanted within 30 min, the success rate has been reported to be as high as 90%.55
It should be noted that it is not always the anesthesiologist who is responsible for dental damage in the operating room. Surgeons, such as otolaryngologists, may inadvertently cause such an injury during rigid laryngoscopy. Also, endoscopists and bronchoscopists have caused trauma to a patient’s dentition. Determining which practitioner was directly involved with the dental trauma is suggested before a discussion with the patient.
Once the patient is sufficiently awake, a discussion of the perioperative dental incident should occur. Patients are more likely to become upset if they feel that this incident has been ignored or practitioners have refused to acknowledge any responsibility. Facilitating prompt attention to the dental damage before the patient’s discharge will ameliorate convenience and reduce expenses. At some health care facilities, dental clinics are on site that can provide an immediate assessment of the injury and suggest appropriate treatment. Due to the relatively small financial payout for repair, it is often not worth the time or expense to prolong a legal dispute over the incident.
If the injury has not been resolved before discharge, patients will typically seek dental treatment with their private dentist and submit a claim for reimbursement. In the case of patients who have not visited a dentist in several years, the treatment plan may also address some unrelated chronic intraoral conditions, culminating in a significantly increased cost estimate. Extractions, periodontal therapy, insertion and restoration of implants, crown and bridge fabrication, etc. may all be necessary measures for improving a patient’s overall dental condition. A reasonable reimbursement by the health care facility should only include the repair costs of the dental damage that was sustained perioperatively. A mediator, such as a hospital risk management member, can investigate patients’ claims of dental injuries; however, ideally the reimbursement process should include an independent dentist to evaluate the treatment plan and fees. Rather than relying upon the assessment of the injury by an administrator, review of the claim by a more appropriately educated individual can ensure an impartial judgment. It also diminishes the potential for the payer to be financially responsible for any extraneous treatment and inflated costs.
Return to the table of contents
Conclusions
Return to the table of contents
Perioperative dental damage is one of the most common anesthesia-related adverse events leading to claims. Understanding and recognizing the multiple risk factors associated with such injuries leads to prevention. During the preoperative evaluation, information about the patient’s intraoral soft and hard tissues should be obtained by the anesthesiologist. Adoption of a more extensive intraoral examination into one’s preoperative evaluation is not suggested for most patients, but in some instances a "hands-on" examination of the patient’s dental status is recommended to properly appreciate any vulnerable teeth or soft tissues. Patients exhibiting very poor oral hygiene and chronic oral neglect may be harboring an unknown odontogenic infection that can compromise surgical outcome, and those with known mobile teeth are at an increased risk for tooth avulsion and/ or aspiration. Decayed, restored, or periodontally involved teeth are more susceptible to becoming damaged perioperatively than a natural dentition. A preoperative discussion with the patient of the risk of dental injury and clear documentation can significantly reduce the magnitude of postoperative disputes and costs. If indicated, securing loose teeth can help reduce dental injury, as will other preventive measures, such as careful mouth opening, laryngosope placement, suctioning, and extubation maneuvers. Following an incident of perioperative dental damage, the goal is to obtain an immediate assessment and provide a fair reimbursement for treating the injury. Enhancing one’s awareness of the various perioperative dental considerations described in this article can minimize costs, while improving anesthetic outcome and patient satisfaction.
Return to the table of contents
Footnotes
Return to the table of contents
Accepted for publication November 18, 2008.
There are no financial relationships between the author of the article and any commercial party.
Return to the table of contents
References
Return to the table of contents
[1]. Lockhart PB, Feldbau EV, Gabel RA, Connolly SF, Silversin JB. Dental complications during and after tracheal intubation. J Am Dent Assoc 1986;112:480–83
[2]. Warner M, Benenfeld S, Warner M, Schroeder D, Maxson P. Perianesthetic dental injuries: frequency, outcomes, and risk factors. Anesthesiology 1999;90:1302–5
[3]. Newland MC, Ellis SJ, Peters KR, Simonson JA, Durham TM, Ullrich FA, Tinker JH. Dental injury associated with anesthesia: a report of 161,687 anesthetics given over 14 years. J Clin Anesth 2007;19:339–45
[4]. Chen JJ, Susetio L, Chao CC. Oral complications associated with endotracheal general anesthesia. Anaesth Sinica 1990;28:163–69
[5]. Gaiser RR, Castro AD. The level of anesthesia resident training does not affect the risk of dental injury. Anesth Analg 1998;87:255–57
[6]. Givol N, Gershtansky Y, Halamish-Shani T, Taicher S, Perel A, Segal E. Perianesthetic dental injuries: analysis of incidence reports. J Clin Anesth 2004;16:173–76
[7]. Owen H, Waddell-Smith I. Dental trauma associated with anesthesia. Anaesth Intensive Care 2000;28:133–45
[8]. Burton J, Baker A. Dental damage during anaesthesia and surgery. Anaesth Intensive Care 1987;15:262–68
[9]. Brosnan C, Radford P. The effect of a toothguard on the difficulty of intubation. Anaesthesia 1997;52:1011–14
[10]. Cass NM. Medicolegal claims against anaesthetists: a 20 year study. Anaesth Intensive Care 2004;32:47–58
[11]. Chopra V, Bovill JG, Spierdijk J. Accidents, near accidents and complications during anaesthesia. Anaesthesia 1990;45:3–6
[12]. Holzer JF. Current concepts in risk management. Anesthesiol Clin North Am 1984;22:91–102
[13]. Kain Z. The National Practitioner Data Bank and malpractice payment. Anesth Analg 2006;103:646–49
[14]. Chadwick RG, Lindsay S. Dental injuries during general anaesthesia. Br Dent J 1996;180:255–58
[15]. Chidyllo SA, Zukaitis JA. Dental examinations prior to elective surgery under anesthesia. NY State Dent J 1990;56:69–70
[16]. Lee J, Choi J, Lee Y, Lee Y, Kim E, Kwon O, Hastings R. The Callander laryngoscope blade modification is associated with a decreased risk of dental contact. Can J Anaesth 2004;51:181–84
[17]. Chadwick RG, Lindsay SM. Dental injuries during general anaesthesia: can the dentist help the anaesthetist? Dent Update 1998;25:76–8
[18]. Vogel C. Dental injuries during general anaesthesia and their forensic consequences. Anesthetist 1979;28:347–49
[19]. Bucx M, Snijders C, van Geel R, Robers C, van de Giessen H, Erdmann W. Forces acting on the maxillary incisor teeth during laryngoscopy using the Macintosh laryngoscope. Anaesthesia 1994;49:1064–70
[20]. Rosenberg MB. Anesthesia-induced dental injury. Int Anesthesiol Clin 1989;27:120–25
[21]. Aromaa U, Pesonen P, Linko K, Tammisto T. Difficulties with tooth protectors in endotracheal intubation. Acta Anaesthesiol Scand 1988;32:304–7
[22]. Wheeler RC. Dental anatomy, physiology and occlusion. Toronto: JB Lippincott, 1974;3–24
[23]. Shafer WG, Hine MK, Levy BM. Oral pathology. Toronto: WB Saunders, 1983:406–78
[24]. Baum L, Lund MR, Philips RW. Textbook of operative dentistry. Toronto: WB Saunders, 1981:12–27
[25]. Boehm TK, Scannapieco FA. The epidemiology, consequences and management of periodontal disease in older adults. J Am Dent Assoc 2007;138suppl:26S–33S
[26]. Carranza FA. Glickman’s clinical periodontology. Toronto: WB Saunders, 1984:1–62
[27]. Carranza FA. Glickman’s clinical periodontology. Toronto: WB Saunders, 1984:192–299
[28]. White A, Kander P. Anatomical factors in difficult direct laryngoscopy. Br J Anaesth 1975;47:468–74
[29]. Yasny JS, Silvay G. The value of optimizing dentition before cardiac surgery. J Cardiothorac Vasc Anesth 2007;21:587–91
[30]. Johnson A, Lockie J. Anaesthesia and dental trauma. Anaesth Intensive Care 2005;6:271–2
[31]. Bartlett DW, Smith BG. Etiology and management of tooth wear: the association of drugs and medicaments. Drugs Today 1998;34:231–9
[32]. Bartlett DW. The role of erosion in tooth wear: aetiology, prevention and management. Int Dent J 2005;55(suppl 1):277–84
[33]. Scully C. Drug effects on salivary glands: dry mouth. Oral Dis 9:165–76
[34]. Ship JA, Hu K. Radiotherapy-induced salivary dysfunction. Semin Oncol 2004;31(suppl 18):29–6
[35]. Shiboski CH, Hodgson TA, Ship JA, Schiodt M. Management of salivary hypofunction during and after radiotherapy. Oral Surg Oral Med Oral Path Oral Radiol Endod 2007;103(suppl 66):e1–e19
[36]. Clockie C, Metcalf I, Holland A. Dental trauma in anaesthesia. Can J Anaesth 1989;36:675–80
[37]. Misra S, Tahmassebi JF, Brosnan M. Early childhood caries—a review. Dent Update 2007;34:556–58
[38]. Tooth eruption: the primary teeth. J Am Dent Assoc 2005; 136:1619
[39]. Windsor J, Lockie J. Anaesthesia and dental trauma. Anaesth Intensive Care 2008;9:355–7
[40]. Herlich A, Garber JG, Orkin FK. Complications in anesthesiology. 2nd edition. (Dental and salivary gland complications). Philadelphia: Lippincott-Raven Publishers, 1996:163–74
[41]. Gatt SP, Aurisch J, Wong K. A standardized, uniform and universal dental chart for documenting state of dentition before anaesthesia. Anaesth Intensive Care 2001;29:48–50
[42]. Al-Quran FA, Al-Dwalri ZN. Torus palatines and torus mandibularis in edentulous patient. J Comtemp Dent Pract 2006;7:112–9
[43]. Small RH, Ganzberg SI, Schuster AW. Unsuspected temporomandibular joint pathology leading to a difficult endotracheal intubation. Anesth Analg 2004;99:383–5
[44]. Bizzarri D, Giuffrida J. Improved laryngoscope blade designed for ease of manipulation and reduction of trauma. Anesth Analg 1958;37:231–2
[45]. Kimberger O, Fischer L, Plank C, Mayer N. Lower flange modification improves performance of the Macintosh, but not the Miller laryngoscope blade. Can J Anaesth 2006; 53:595–601
[46]. Watanabe S, Suga A, Asakura N, Takeshima K, Kimura T, Taguchi N. Determination of the distance between the laryngoscope blade and the upper incisors during direct laryngoscopy: comparisons of a curved, an angulated straight, and two straight blades. Anesth Analg 1994;79:638–41
[47]. Bito H, Nishiyama T, Higarhizawa T, Sakai T, Konishi A. Determination of the distance between the upper incisors and the laryngoscope blade during laryngoscopy: comparisons of the McCoy, the Macintosh, the Miller, and the Belscope blades. Masui 1998;47:1257–61
[48]. Salisbury P 3rd, Curtis J Jr, Kohut R. Appliance to protect maxillary teeth and palate during endoscopy. Arch Otolaryngol 1984;110:106–7
[49]. Skeie A, Schwartz O. Traumatic injuries of the teeth in connection with general anaesthesia and the effect of use of mouthguards. Endod Dent Traumatol 1999;15:33–6
[50]. Solazzi RW, Ward RJ. The spectrum of medical liability cases. Int Anesthesiol Clin 1984;22:43–59
[51]. Pollard BJ, O’Leary J. Guedel airway and tooth damage. Anesth Intensive Care 1981;9:395
[52]. Dornette WH, Hughes BH. Care of the teeth during anesthesia. Anesth Analg 1959;38:206–15
[53]. Dornette WH. Care of the teeth during endoscopy and anesthesia. Clin Anesth 1972;8:213–17
[54]. Neustein S, Beicke M. Ingestion of a fixed partial denture during general anesthesia. Anesth Prog 2007;54:50–1
[55]. Kainuma M, Yamada M, Miyake T. Early application of the cross-suture splint to teeth avulsed at tracheal intubation [Letter]. Anesthesiology 1996;84:1516
Explorative laparatomy for diagnosis of abdominal painful syndromes in HIV positive patients.
Angelici A, Palumbo P, Piermattei A, Toma L, Romani R, Delia S; International Conference on AIDS.
Int Conf AIDS. 1993 Jun 6-11; 9: 447 (abstract no. PO-B19-1869).IV Surgical Department, Univ. La Sapienza, Rome, Italy.
Abdominal pain may often represent the first symptom of important opportunistic conditions related to HIV infection. In particular, infections by atypical mycobacteria, mainly belonging to the Mycobacterium avium complex (MAC), are a frequent cause of abdominal painful syndromes which usually arise difficult diagnostic and therapeutic problems. Diagnosis is usually made on the basis of a positive blood culture, but sometimes ecography or TC-guided biopsy of deep lymph nodes (retroperitoneal, spleen or liver hilum, mesenteric root) may be required. However, the material obtained with the latter approach may not be adequate for diagnosis and surgery may be the only mean to the identification of the etiologic agent. In the present study we report data about 4 patients with MAC infection in which diagnosis could only be made by surgical approach. Two patients underwent emergency explorative laparotomy for acute abdomen and MAC intestinal infection was diagnosed; in the other 2 cases elective surgery was performed and biopsy of lymph nodes revealed MAC lymphadenopathy. Diagnostic imaging limits and surgical risks in these patients, who often will not take much advantage from specific treatment, will be discussed.
http://gateway.nlm.nih.gov/MeetingAbstracts/ma?f=102204836.html